Adults living with the most common inherited heart disease (hypertrophic cardiomyopathy or HCM) now have access to Australia’s first targeted treatment for the disease on the Pharmaceutical Benefits Scheme (PBS).
CAMZYOS (mavacamten) for obstructive HCM, targets the underlying cause of the disease, in which abnormal, progressive thickening of the left heart muscle (ventricle), makes it hard for the heart to pump blood around the body.
Obstructive HCM is caused by the walls of the left ventricle becoming so thickened, that they obstruct blood flow out of the heart, causing it to stiffen, making it harder for the heart to beat.
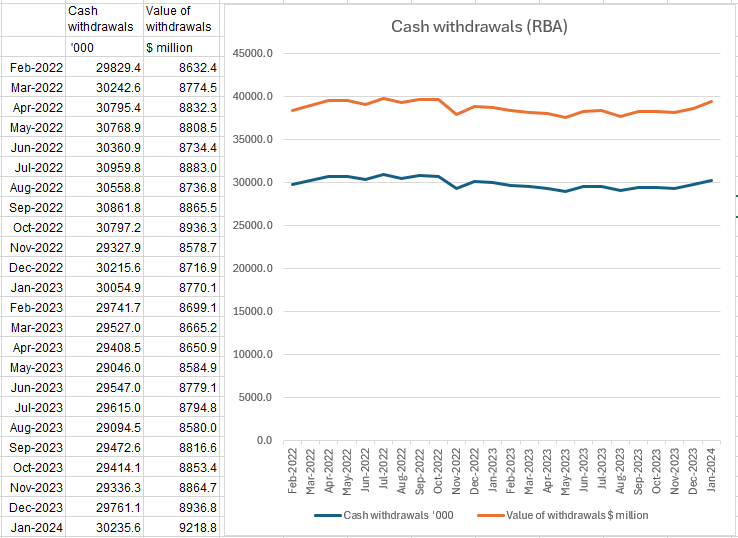
New Australian research reveals more than 60,000, or one in 400 Aussies are living with HCM, with many cases of the disease remaining undiagnosed, and under-treated.
The disease may cause chest pain, shortness of breath with physical exercise, dizziness, loss of consciousness, and abnormal heart rhythms, and lead to heart failure and premature death in people of all ages.
According to Cardiologist, Director of the HCM Clinic, The Alfred Hospital, and Director of Victoria Heart, Dr Andris Ellims, Melbourne, there is an unmet need for non-invasive treatment options for obstructive HCM that target the underlying cause of the disease.
“We need more treatment options to support Australians living with this progressive, debilitating disease,that is often difficult to diagnose, and treat.
“The disease can cause chest pain, shortness of breath with physical exercise, dizziness, loss of consciousness and abnormal heart rhythms,” said Dr Ellims.
“HCM can also lead to heart failure and premature death in people of all ages.”
For some, HCM can be a debilitating and life-changing disease, causing impaired function and reduced quality of life.
Some of the most commonly reported effects of HCM symptoms include limited physical activity, emotional stress, such as feeling anxious or depressed, and a compromised work life. Having another treatment option available to this patient community is therefore, welcome news for clinicians and patients alike.
When law firm partner and owner, and father-to-three, Daniel, 58, Sydney, was diagnosed with obstructive HCM at 44 years of age, it came as quite a shock, given he had no known family history of the disease.
“I was working hard, caring for three young kids, trying to keep fit, and to socialise, but I would get extremely tired and cranky. I was no fun to be around. Then I was diagnosed with HCM in April 2010,” said Daniel.
“I was changing my insurance and had to go for an insurance medical. I remember getting on a treadmill and having an exercise cardiogram. I returned home, and went out for a walk with my wife when the doctor rang and told me I had cardiomyopathy, and had to see him immediately.
“As my symptoms grew worse, I began to worry about whether I would be able to function moving forward, and the potential implications for my young family, and I,” Daniel said.
“I had to alter my lifestyle significantly and get comfortable living with the disease. I was forced to reduce my expectations, and to amend my lifestyle to accommodate my symptoms.”
Chief Executive Officer of Heart Support Australia – Australia’s largest peer support network for Australians affected by heart disease, and Clinical Senior Lecturer, University of Sydney and University of Adelaide, Dr Christian Verdicchio, Adelaide, welcomed today’s reimbursement of a new treatment option for those living with obstructive HCM.
“Obstructive hypertrophic cardiomyopathy is a common genetic heart condition affecting the muscles of the heart. To date there have been limited medical treatment options for this disease, that can be debilitating, and lead to sudden death.
“Knowing Australians now have access to a new treatment option, is important news for this patient group,” said Dr Verdicchio.
“We thank the Federal Minister for Health, and the Department of Health for this important approval which will no doubt improve the lives of those living with HCM.”
BMS Australia and New Zealand Medical Director, Dr Meredith Edwards, Melbourne, said the PBS listing of CAMZYOS represents a significant step forward for the Australian obstructive HCM patient community.
“The reimbursement of this first-of-its kind medicine may help to address an unmet need for Australians living with symptomatic NYHA class II-III obstructive HCM.”